The answer is (c) Resist large changes in pH upon addition of H+ only. The statement that the buffer solution will only resist large changes in the pH level upon the addition of Hydrogen ions is false.
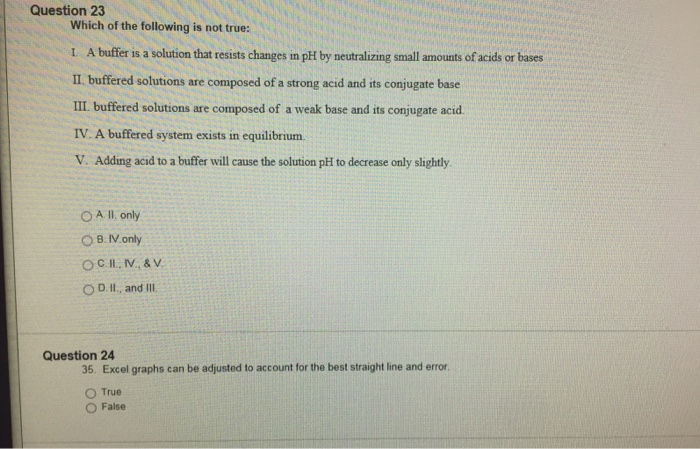
Which is not true about buffering?
The answer is (c) Resist large changes in pH upon addition of H+ only. The statement that the buffer solution will only resist large changes in the pH level upon the addition of Hydrogen ions is false.
What is true about buffering?
Buffer solutions have the capacity to react with small amounts of added acid or base without affecting the hydrogen ion concentration of the solution. Thus, the buffer solutions help to keep the pH value constant in a chemical reaction.
What is incorrect about buffer solution?
Solution : Buffer solutions does not show any change in pH on addition of small amount of acid or base.
Which one is not correct about buffer mixture?
A buffer solution either is a mixture of a weak acid and its salt with strong base or a mixture of a weak base and its salt with strong acid. Hence, clearly CH3COONH4 is not a buffer solution.
How do buffers work?
How do buffers work? Buffers work by neutralizing any added acid (H+ ions) or base (OH- ions) to maintain the moderate pH, making them a weaker acid or base.
What does it mean by buffering?
Buffering is the process of preloading data into a reserved area of memory that’s called a buffer. In the context of streaming video or audio, buffering is when the software downloads a certain amount of data before it begins playing the video or music.
Which of the following is correct about buffer solution?
Its pH changes very little when a small or moderate amount of strong acid or base is added to it and thus it is used to prevent changes in the pH of a solution. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications.
Which of the following is true regarding buffer capacity?
Which of the following is true regarding buffer capacity? Buffer capacity is the ability of a system to avoid changes in pH as long as the amount of H+ or OH- ion added does not exceed the ability of the buffer system to neutralize it.
What is the purpose of buffer class in node?
The Buffer class in Node. js is designed to handle raw binary data. Each buffer corresponds to some raw memory allocated outside V8. Buffers act somewhat like arrays of integers, but aren’t resizable and have a whole bunch of methods specifically for binary data.
What is the pH of a neutral solution?
The range goes from 0 – 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.
Which of the following is not are not a buffer solution?
Solution : Buffer solutions can be obtained by mixture a weak acid with its salt formed with a strong base or by mixing a weak base with its salt formed with a strong acid.
As `HClO_4` is a strong acid, therefore equimolar mixture of `HClO_4` and its salt `KClO_4` is not a buffer solution.
Which among the following is not a property of buffer solution?
The pH of a buffer solution does not change on keeping for a long time. Thus, pH of a buffer solution remains constant on keeping form a long time. Thus, the statement it does not have a definite pH is incorrect i.e. it is not a property of a buffer solution.
Which of the following system is not a buffer system?
A buffer solution is consists of a weak acid and its conjugate base or vice versa. In option B NaClO4/HClO4 is not a buffer because HClO4 is a strong acid.
Which of these is not a type of buffer?
D. NH4NO3. Hint: A buffer solution either is a mixture of a weak acid and its salt with strong base or a mixture of a weak base and its salt with strong acid. So the solution which is not satisfying this condition is not a buffer solution.
What is buffering Mcq?
Explanation: The buffer is an area that is used to store data temporarily which can be used to compensate the timing problems.
What does a buffer consists of?
In chemistry, the definition of a buffer is a solution that can resist pH change upon the addition of an acid or a base. It consists of a solution of a weak acid and its conjugate base, or vice versa. A buffer is an extremely useful solution used in acid base chemistry.
What is the purpose of a file buffer?
A file buffer is the temporary image of the file that you can edit. You can edit the file buffer without affecting the original file, until you save it using the Save command. The File > Save command writes the file buffer contents back over the original file. This is the only time the original file is changed.
Which is not true about buffering?
The answer is (c) Resist large changes in pH upon addition of H+ only. The statement that the buffer solution will only resist large changes in the pH level upon the addition of Hydrogen ions is false.
Which one is not correct about buffer mixture?
A buffer solution either is a mixture of a weak acid and its salt with strong base or a mixture of a weak base and its salt with strong acid. Hence, clearly CH3COONH4 is not a buffer solution.
Which of the following is characteristic of a buffer?
Buffers have the capability to resist change in pH. The pH will not change if a small amount of concentrated or strong acid or base is added. This is because a buffer solution consists of a conjugate acid-base pair that neutralizes the acid or base added and resists the change in the pH.
Which of the following is characteristic of a buffer quizlet?
A buffer contains which of the following? A significant amount of a weak acid and its conjugate base.